Br Lose Or Gain Electrons
How can you tell if an element wants to gain or lose electrons?
The answer to your question is below. Explanation: Metals lose electrons and Nonmetals gain them. Ra is in group IIA it will lose electrons, the ion will be Ra⁺². Br is in group VIIA it will gain electrons, the ion will be Br⁻¹. In is in group IIIA it will lose electrons, the ion will be In⁺³.
1 Answer
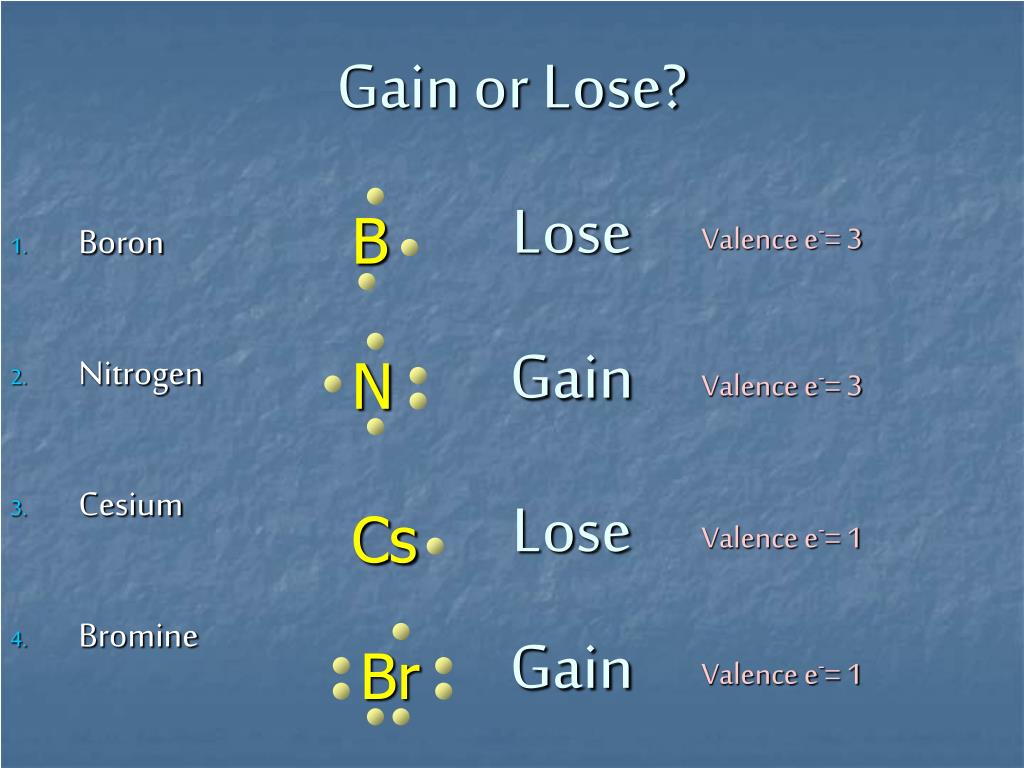
Explanation:
Ion Worksheet Element # Valence Electrons # Electrons to Gain # Electrons to Lose Ion Formed/ Name Li 1 None 1 Li +1/ cation N 5 3 None N – 3/ anion O 6 2 None O-2/anion Ca 2 None 2 Ca+2/cation Br 7 1 None Br-1/anion S 6 2 None S-2/anion Cl 7 1 None Cl-1/anion K 1 None 1 K+1/cation Mg 2 None 2 Mg+2/cation Be 2 None 2 Be+2/cation Element Name. Nitrogen atoms gain 3 electrons and form the nitride ion, N3. Nitrogen atoms also form covalent bonds where they share 3 electrons and do not become ions. Bromine atoms gain 1 electron and form. The valence electron for phosphorus is 5. To achieve an octet electron arrangement, it needs to lose 5 electrons or gain 3 electrons. It is easier to gain 3 electrons than to lose 5 electrons. So phosphorus has to gain 3 electrons. If a molecule is oxidized, does it gain or lose energy? Oxidation occurs when a molecule loses an electron or increases its oxidation state. When a molecule is oxidized, it loses energy. In contrast, when a molecule is reduced, it gains one or more electrons.
In general, metals will lose electrons to become a positive cation and nonmetals will gain electrons to become a negative anion. Hydrogen is an exception, as it will usually lose its electron. Metalloids and some metals can be can lose or gain electrons.
This is not always true, as elements such as nitrogen can lose electrons to become positive. When an ionic compound forms, the more electronegative element will gain electrons and the less electronegative element will lose electrons.
Elements That Lose Electrons
Related questions
Chemistry
Which atom has the smallest valence p atomic orbital? -Carbon -Nitrogen -Sulfur Why? A. That atom has the highest electronegativity. B. That atom has the fewest number of electrons. C. That atom does not have electrons in p
chemistry
elements below, indicate how each elements’ valence electrons act in a chemical reaction. Insert an L if an element loses electrons, a G if the element gains electrons, an E if the element can either lose or gain electrons, and
Ap Chem
explain each of the following observations using principles of atomic stucture and/or bonding. a] potassium has a lower first-ionization engergy than lithium. b] the ionic radius of N3- is larger than that of O2-. c] a calcium
Chemistry
Why would two atoms form a covalent bond instead of an ionic bond? Both atoms in a bond have similar electronegativity; thus, neither atom is willing to transfer their electrons. Instead, the atoms both share electrons to satisfy
Atoms Gain Or Lose Electrons
You can view more similar questions or ask a new question.